How Mara works.
The Mara system uses a unique energy source: water vapor. By filling the uterine cavity with naturally expansive water vapor, Mara gently treats more women with varying uterine anatomies*, does not require general anesthesia, and most importantly, safely and effectively reduces heavy menstrual bleeding.1
*With a broad range of anatomical conditions: uterine cavity lengths greater than 10 cm (up to 12 cm), cavities with certain type of myomas up to 4 cm, and in the presence of Essure®
2-minute active treatment time*
*As part of a 4-minute procedure. Total time in office may vary and will include preparation and post-procedure observation.
Designed Specifically for Your Office
Console
- Fully automated water vapor delivery system
- Intuitive, user-friendly interface
- IntegrityPro™ technology confirms cavity integrity and proper device placement prior to water vapor delivery
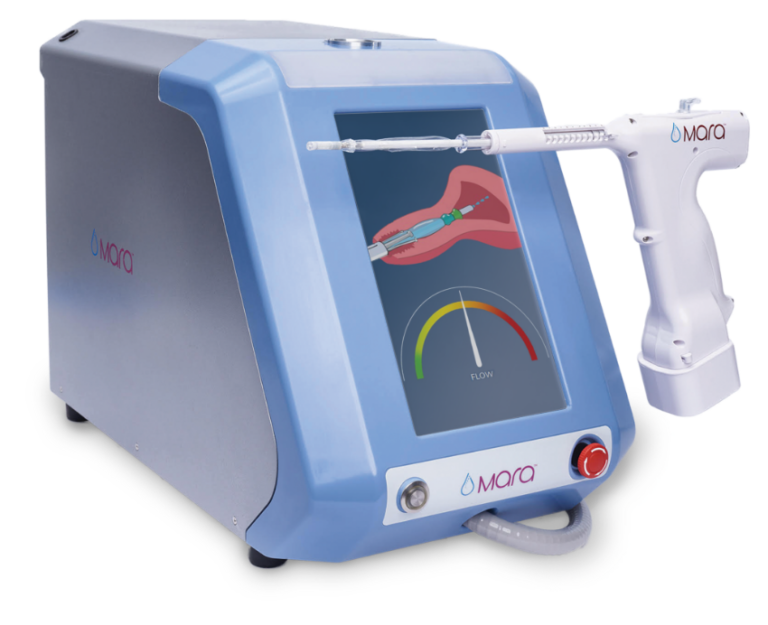
Water Vapor Probe
- Slender, soft, flexible tip
- Cervical collar aids in device placement just beyond the internal cervical os
- SmartSeal™ technology provides redundant cervical sealing with its triple balloon sealing system and continuous temperature monitoring
Indication for Use
The Mara Water Vapor Ablation System is indicated to ablate the endometrial lining of the uterus in premenopausal women with menorrhagia (excessive uterine bleeding) due to benign causes for whom childbearing is complete.
Important Safety Information
Mara Water Vapor Ablation System is indicated to ablate the endometrial lining of the uterus in premenopausal women with menorrhagia due to benign causes for whom childbearing is complete. Pregnancy following the Mara procedure can be dangerous. The Mara procedure is contraindicated for those who have suspected uterine cancer, pre-malignant conditions of the endometrium, endometrial hyperplasia, prior classical cesarean section, transmural myomectomy (including myomectomy performed immediately prior to Mara procedure), taking medications that thin the uterine muscle, uterine length <6cm, prior endometrial ablation, active genital or urinary tract infection, PID, or systemic bacterial infection, IUD currently in place, suspected hydrosalpinx or undiagnosed abnormal vaginal bleeding. Mara is not a sterilization procedure. Rare but serious risks include, but are not limited to, perforation, infection, thermal injury, and serious complications in future pregnancies. Temporary side effects may include cramping, nausea, abdominal pain and distension, vomiting, vaginal infection, and endometritis. Inform patients to contact you if they experience a possible side effect related to Mara. For detailed benefit and risk information, please consult the IFU.
References
- Mara Water Vapor Ablation System Instructions For Use

