Water Vapor Probe Tip
- Slender, soft, flexible tip
- Does not need to come in contact with the uterine fundus
The Mara™ Water Vapor Ablation System enables you to help reduce heavy menstrual bleeding in more women* in the convenience of your office.1
*With a broad range of anatomical conditions: uterine cavity lengths greater than 10 cm (up to 12 cm), cavities with certain type of myomas up to 4 cm, and in the presence of Essure®


Mara utilizes the expansive properties of water vapor to conform to each woman’s unique uterus, enabling you to treat women who were traditionally not indicated for endometrial ablation,1 including women with:
32% of patients effectively treated in the Mara Pivotal Trial may not be appropriate candidates with other ablation options.1
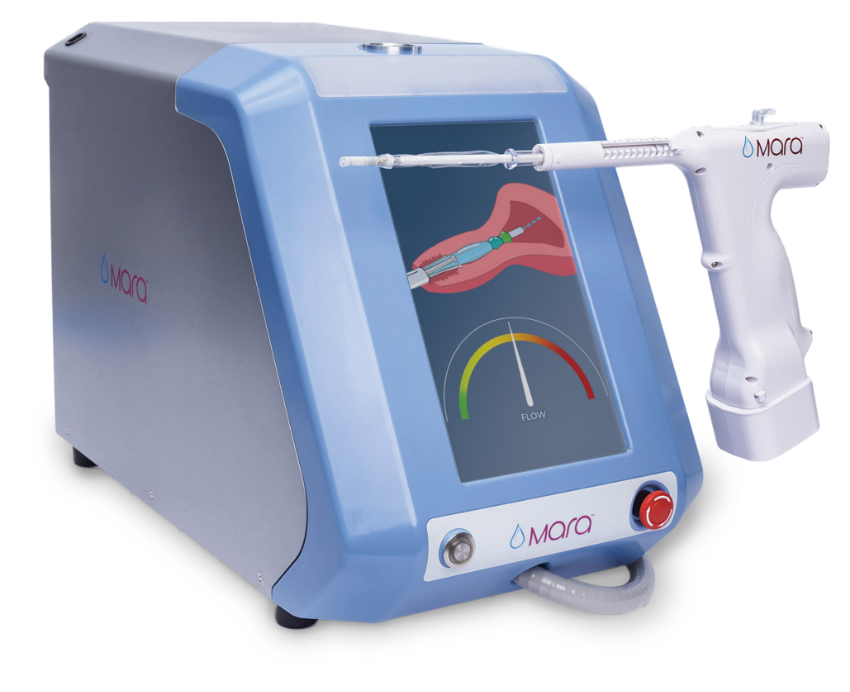
Mara is the first FDA-approved water vapor endometrial ablation treatment specifically designed for your office without the need for general anesthesia.
*Total time in office may vary and will include preparation and post-procedure observation.
Mara helps you manage post-ablation patients by preserving cavity access.3
The PACE clinical study demonstrated that a mean of 4 years following water vapor ablation treatment, Mara preserved an accessible and evaluable uterine cavity in 90% of the patients studied with minimal findings of severe adhesions.
Your ability to treat patients with confidence is our top priority. With Mara, automated safety features provide a double pre-procedure safety assessment to ensure uterine cavity sealing and provide real-time monitoring throughout the procedure.1 Key safety features include:
Mara significantly reduces heavy menstrual bleeding and improves quality of life as demonstrated in an international, multicenter pivotal clinical study that enrolled 155 women. 79% of patients met the primary endpoint of the study with a PBLAC score of ≤ 75 at 1 year.1
of women experienced an improvement in quality of life2
Based on patient reported Menorrhagia Impact Questionnaire (MIQ) scores
of women would recommend Mara to a friend1
hysterectomy rate at 3 years1
The Mara Water Vapor Ablation System is indicated to ablate the endometrial lining of the uterus in premenopausal women with menorrhagia (excessive uterine bleeding) due to benign causes for whom childbearing is complete.
Mara Water Vapor Ablation System is indicated to ablate the endometrial lining of the uterus in premenopausal women with menorrhagia due to benign causes for whom childbearing is complete. Pregnancy following the Mara procedure can be dangerous. The Mara procedure is contraindicated for those who have suspected uterine cancer, pre-malignant conditions of the endometrium, endometrial hyperplasia, prior classical cesarean section, transmural myomectomy (including myomectomy performed immediately prior to Mara procedure), taking medications that thin the uterine muscle, uterine length <6cm, prior endometrial ablation, active genital or urinary tract infection, PID, or systemic bacterial infection, IUD currently in place, suspected hydrosalpinx or undiagnosed abnormal vaginal bleeding. Mara is not a sterilization procedure. Rare but serious risks include, but are not limited to, perforation, infection, thermal injury, and serious complications in future pregnancies. Temporary side effects may include cramping, nausea, abdominal pain and distension, vomiting, vaginal infection, and endometritis. Inform patients to contact you if they experience a possible side effect related to Mara. For detailed benefit and risk information, please consult the IFU.